Determining Atomic Weight
Concept Question: How do scientists determine the atomic weight of an element?
For any element, not all isotopes exist in the same amounts in nature. Open the IUPAC Periodic Table of the Elements and Isotopes Learning Tool, click on carbon and then select the 'more information' tab. You will see that carbon has 15 isotopes, but in a sample, 98.93 % of carbon atoms exist as carbon-12. The only other stable isotope of carbon, carbon-13, makes up the other 1.07 % of that sample. There is one long-lived radioisotope of carbon, carbon-14, which exists in trace amounts in nature. The other 12 isotopes have half-lives less than 1 second, and don't exist long enough to show up as significant portions of naturally occurring carbon atoms.
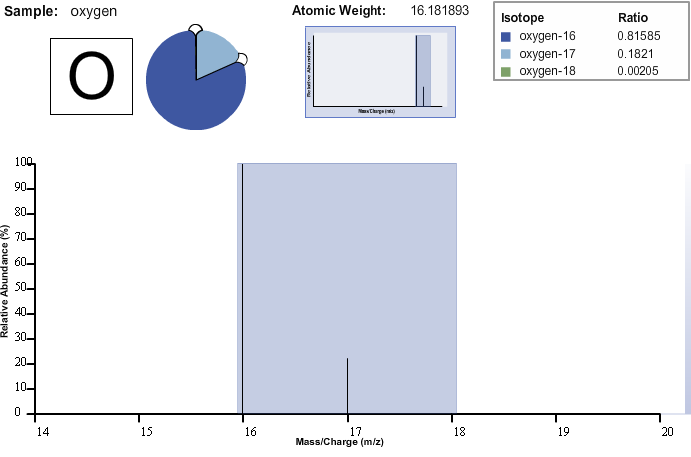 The abundances of the various
isotopes of an element are also determined using mass spectrometry. Open the Mass Spectrometer Learning Tool and select oxygen from the options menu. This time,
adjust the ratios of the various isotopes of oxygen by clicking on the lines within the pie chart and dragging them to whatever values you desire. Then, scan the sample. When the scan is complete, view the spectrum. Click on
the peaks to observe the relative intensities of each isotope. Notice that the heights of the peaks correspond to the relative abundance of each of the isotopes.
The abundances of the various
isotopes of an element are also determined using mass spectrometry. Open the Mass Spectrometer Learning Tool and select oxygen from the options menu. This time,
adjust the ratios of the various isotopes of oxygen by clicking on the lines within the pie chart and dragging them to whatever values you desire. Then, scan the sample. When the scan is complete, view the spectrum. Click on
the peaks to observe the relative intensities of each isotope. Notice that the heights of the peaks correspond to the relative abundance of each of the isotopes.
It is a common to automatically set the highest intensity peak to 100 % and express the other peaks as percentages of the largest peak. Therefore, if a mass spectrum shows one peak with an intensity of 100 % and another peak with an intensity of 25 %, this means the second isotope is one-fourth as abundant as the first isotope (100:25 = 4:1). Therefore, 80 % of the sample was composed of the first isotope and 20 % consisted of the second isotope.
Previous versions of the Mass Spectrometer Learning Tool scaled their peaks in this way, and you may encounter this approach while working with Mass Spectrometry data elsewhere. As such, it is important that you understand how to translate and interpret the Mass Spectrometry data that you are working with. Make sure you are paying attention to how the data is presented and take note if the data has been scaled.